Eye tolerability of consumer care products
All eyes on the eyes
At proderm Consumer Care, evaluating the irritation potential of test products in and around the human eye is a very common task. We can provide you with ophthalmological and subjective assessments of eye tolerability in any in-home use test of products like e.g. shampoos, facial creams, make-up and decorative cosmetics which can be reasonably expected to come into contact with the eyes.
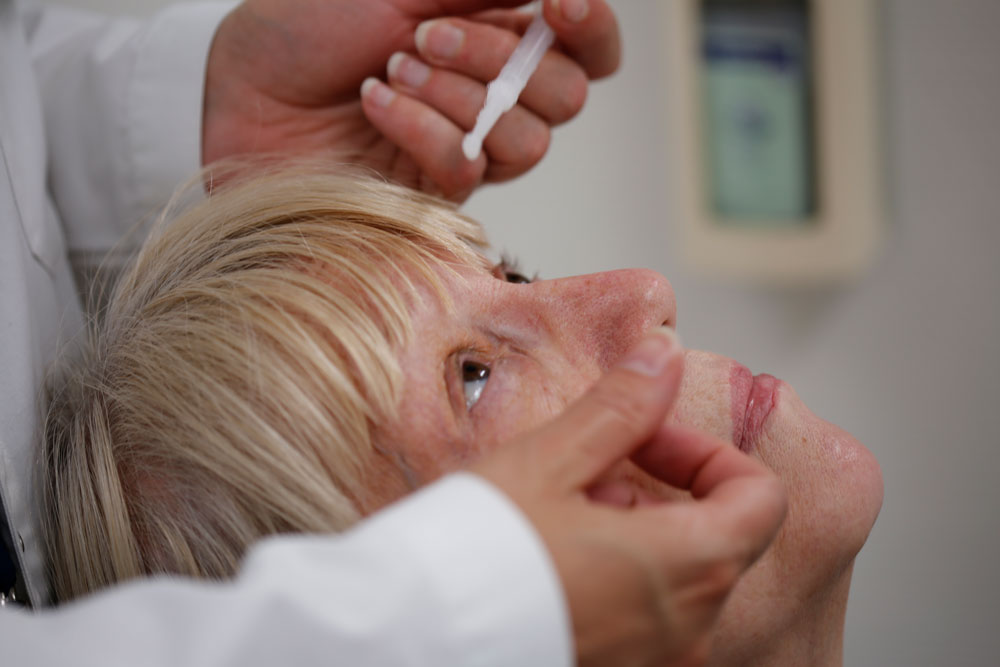
For a closer look on eye irritation potential, we offer eye instillation studies to evaluate the ocular irritation potential of your products or compounds. The test products are instilled directly into the eyes of volunteers under supervision of an ophthalmologist and we use objective and subjective parameters to measure eye irritation. Eye instillation designs start with a range finding phase to identify the best concentration for your specific compound or product. By including a reference product with proven low irritation potential or including contact lens wearers into the panel, eye instillation studies can also be easily adapted to support claims like "no tear" or "suitable for contact lens users"
Objective parameters
In addition to safety and tolerability parameters, we can also measure objective parameters like the tear film break up time and intraocular pressure.
Before the study may be conducted, toxicological data about local skin and mucosa tolerability of the test materials is needed. Please refer to the EU validated test methods.
The following table illustrates a widely used eye irritation test design as an example:
| Eye Instillation test/ Eye Irritation test | |
| Aim | The objective of this study is to evaluate the irritation potential of the test products under the control of an ophthalmologist. |
| Parameters | P1: Ophthalmologic evaluation by Ophthalmologist (lacrimation, bulbar conjunctiva, palpebral conjunctiva and dilation of scleral vessels) |
| P2: Subjective evaluation by Subjects (burning, stinging, itching) | |
| Test product | 2 Test products, application according to the sponsor's instructions. |
| Range Finding | For determination of local tolerance, 3 different doses will be tested per test product in ascending concentrations (1 %, 5 %, 10 %) in 3 subjects each with evaluations after 30 sec., 2 or 3 min., 15 min., 30 min. and 60 min. |
| Test procedure |
|
| Panel | 25 subjects enrolled, aged between 18 and 75 years (f/m, without further in-/ exclusion criteria). |