Products designed to bond to the skin such as patches and tapes are something of a peculiarity in clinical development due to their adhesive properties. In addition to concerns regarding skin tolerance and effectiveness, product adhesion qualities are a third dimension of study that is absent in other products. In this article, we aim to inform developers of patches of the various study and testing opportunities in the related development phases. Please also consider our note at the end of the article regarding the changes that have arisen as a result of the MDR.
- Category
- Consumer Care
- Date
- Share
Determination of adhesive strength
Adhesive strength can be determined using in vitro and in vivo studies. The available methods are DIN EN 1939 in vitro testing or the in vivo adhesive strength measurement method.
The DIN method involves the tested products being investigated on metal (e.g. stainless steel) or a textured surface (double-sided coated adhesive tape) under pre-defined temperatures and angles of removal.
During proderm in vivo testing, the tested products are applied to the backs of the study participants and then removed by machinery. The force applied to pull the patch off is the parameter of adhesive strength. Skin moisture can also be examined in the test environment and a visual evaluation of any reddening or adhesive residues can be performed.
Whichever method is most suitable for you will depend entirely on your individual objectives. Steel and double-sided adhesive tape have entirely different properties to human skin, and the DIN standard is limited in the value of its results.
“Wear study” patch adhesion test
However, the results of the aforementioned in vivo method for measuring adhesive strength cannot provide answers regarding the adhesion of an existing patch. This is due to the non-adhesive component that is integrated into the patch and rests on the wound. The properties of this component can significantly affect the adhesive properties of the patch as a whole.
This is why the performance of a “wear study” is recommended for determining patch adhesion. This study involves the application of an existing whole patch to the skin. It is worn for several days, and trained evaluators judge the adhesion as a percentage and report on any adhesive residues and skin reddening. There is also an evaluation of how comfortable it is to wear and a report on pain sensations when the patch is removed.
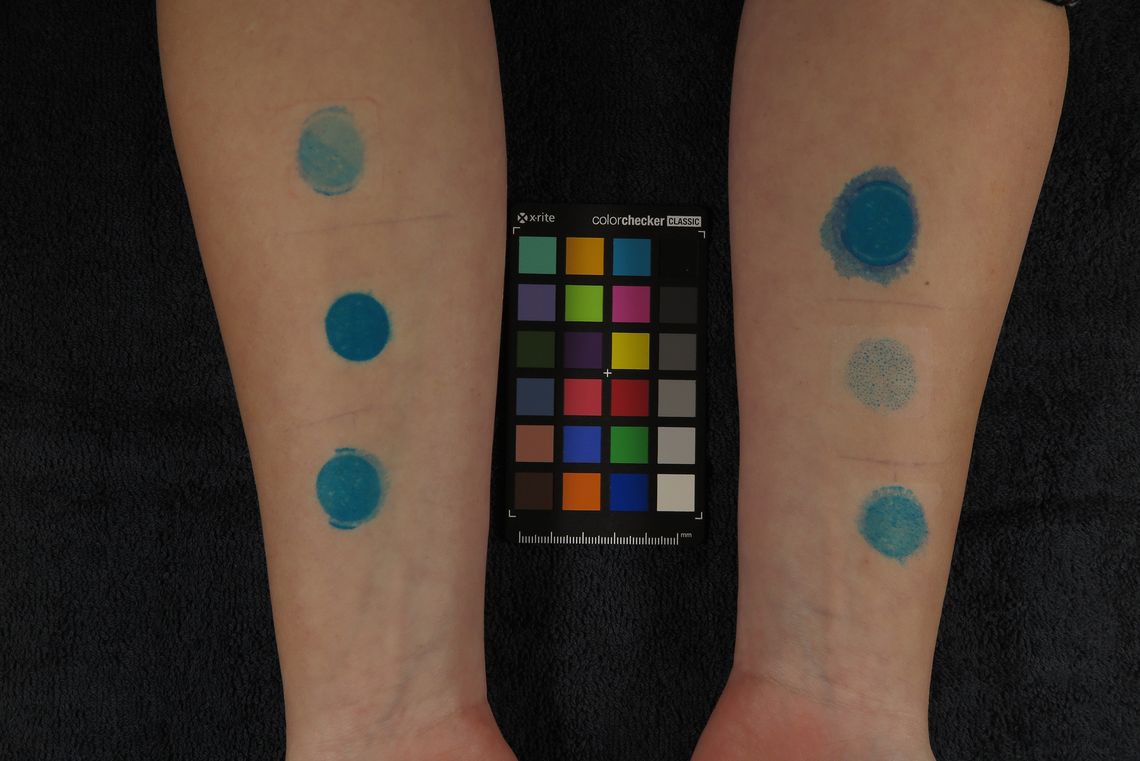
Evaluation of removal of outermost layer of epidermis – “blue dye” test or “skin stripping” study
Manufacturers of patches should examine all of their product’s effects on skin during development – including how pulling the patches off damages the skin. This is especially necessary for patches that are applied repeatedly to a certain spot, like wound patches.
If superficial damage to the skin needs to be studied, the use of the “blue dye” test is recommended. This involves the surface of the skin being coloured blue before the patch is applied. After it has been left on for several hours, the test product is removed and the amount of dye remaining is measured using an instrument. A comparison to a control product provides information on how much of the outermost epidermis has been removed.
On the other hand, the skin-stripping study tests whether the removal of the patch causes damage to the skin barrier. The test product is re-applied each day to an identical area. Transepidermal water loss measurements provide information about the condition of the skin barrier.
Skin tolerance testing
The use of a patch can cause skin irritation. These reactions can be tested in a “wear study” or in a “skin stripping” study. It may also be advisable to conduct a dedicated tolerance study. Such a study is conducted as a patch test under dermatological oversight. Here, the test product is first applied for several hours. The primary skin irritation is then visually assessed by trained evaluators.
Effective wound healing
In addition to the purely physical effect of providing coverage for a wound, a patch can also help with healing properties. The effectiveness of a patch in terms of wound healing can be studied using a variety of methods. For dry wound healing concepts, techniques such as the abrasive wound method or the suction blister method are suitable. Wet wound healing concepts, on the other hand, are tested using the maceration method. A CE label for the test product is mandatory for the performance of wound healing studies.
The abrasive wound method
The abrasive wound method enables studies of a test product’s wound healing properties, for example in comparison to a reference product. First, a sterile brush is used to create a wound. This is then followed by an application phase with the test product, usually lasting 14 days, in which the healing of the wound is documented using digital photographs and visual scoring. The study is analyzed based on a “wound size” parameter using special analysis software.
The suction blister method
The suction blister method is an alternative method for determining the wound healing properties of a test product. The wound is created using a vacuum unit, which generates a suction blister in a test area, and the skin on the blister is removed. This creates a highly standardised wound that heals without leaving a scar.
Maceration
Maceration techniques are used to determine the effectiveness of wet wound healing methods. This model involves the test product being applied to the wound for a longer period of time. A comparison with an untreated wound then allows for conclusions about the effectiveness of the patch intended to support wound healing. Alternatively, a comparison with a dry product or other reference product is also possible.



![[Übersetzen nach: English] Entwicklung von Pflastern](/fileadmin/_processed_/4/b/csm_medizinprodukte_e241baf5a4.jpg)