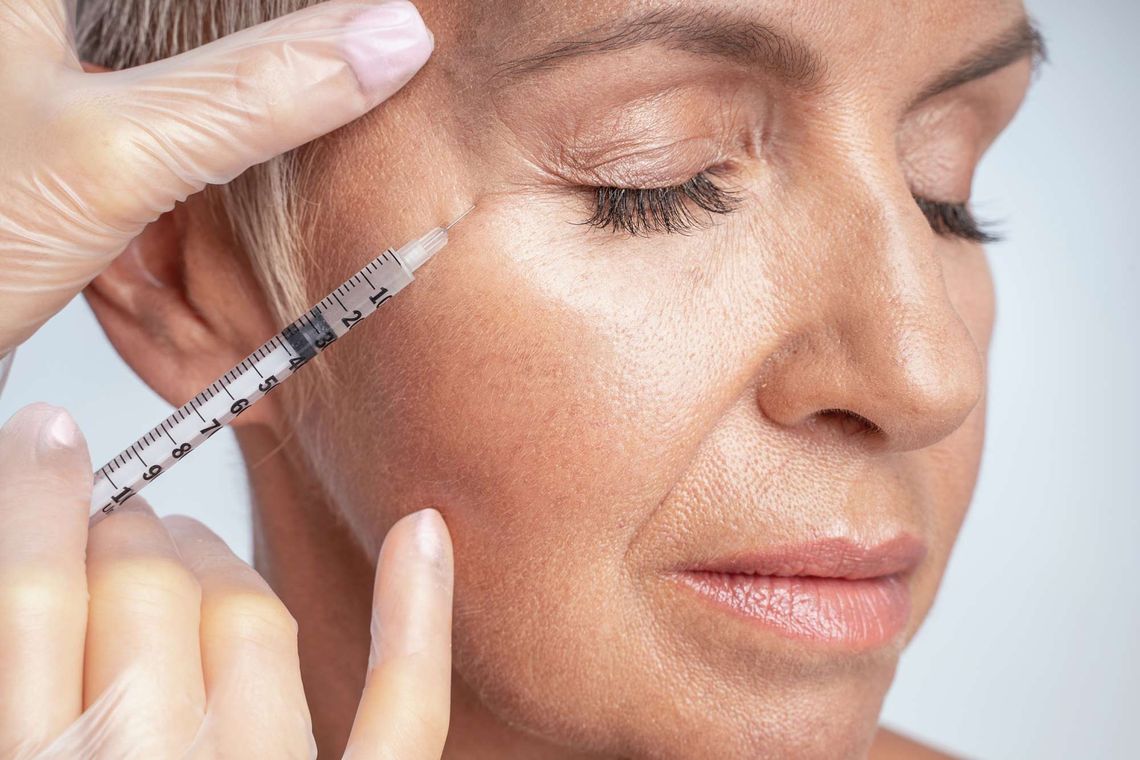
The use of injectables such as botulinum toxin and dermal fillers has become increasingly popular in aesthetic dermatology. In response, EU regulations relating to these products have evolved to safeguard patient safety. This upcoming webinar will delve into the regulatory framework surrounding various types of injectables, as well as the methods used to evaluate their effectiveness and safety.
- Type
- Webinars-on-demand
- Date
- Location
-
Online
This will be a virtual conference. Details about the platform will be provided after completion of the registration.
- Share
Overview
Following questions will be answered within the webinar:
- What is scope of injectables in aesthetic dermatology?
- Which regulations are in place?
- How does the legal framework impact the clinical testing?
- What are the safety aspects one need to consider?
- How is product efficacy evaluated?
- Which technologies can complement subjective assessments of treatment effectiveness?
Registration
Webinars-on-demand
Aesthetic dermatology: Clinical testing of injectables